The Complex Journey of Vaccine Storage: Ensuring Cold Chain Integrity

When you are responsible for storing critical vaccines like the ultra-cold COVID-19 vaccine, medical tissue samples, and other assets stored in medical grade refrigerators or freezers, disaster is always looming — especially when you aren’t at work. Medical and pharmaceutical products can be ruined if the exact temperature is not maintained while in storage. And chances are you need a continuous temperature monitoring device to ensure compliance with regulatory standards.
Vaccines are powerful weapons against disease, but they're also delicate creatures. Much like a rare orchid that needs the perfect environment to thrive, vaccines demand rigorous cold chain management. Ensuring cold chain integrity is no walk in the park - it's a complex journey riddled with pitfalls. But with high stakes – the health and lives of millions – the importance of maintaining this frigid voyage cannot be overstated.
The Importance of the Cold Chain in Vaccine Storage
Vaccine Potency and Stability
The "cold chain" refers to the unbroken refrigerated transportation and storage that vaccines must undergo from manufacturing to administration. Why so cold? It all boils down to stability. The biochemical structures of vaccines can be highly sensitive to temperature variations. Maintaining vaccines within a specific temperature range (usually between 2°C and 8°C) ensures their potency is not compromised.
Global Health Implications
Maintaining cold chain integrity is not only about ensuring the vaccine's effectiveness. It's also a critical aspect of global health. Inefficient vaccine storage and distribution could result in compromised vaccines, leading to ineffective immunization programs. In the worst-case scenario, it can lead to outbreaks of preventable diseases, putting public health at risk.
It is essential to have have a remote temperature monitoring system anywhere these drugs are stored.
However, a cold chain doesn’t have it easy. Cold chains could get disrupted due to the following reasons.
1. Pressure to Meet Cost Efficiencies in Cold Chain Management
2. Lack of Uniform Infrastructure Globally Affecting Cold Chains
3. Impact of Increased Regulations on Cold Chain Management
4. Environmental Impact on Your Cold Chain
5. Supplier Risk in Your Cold Chain
6. Distribution/Delivery Risk in Cold Chain
How to Reduce Risks in Cold Chain Management?
You need a real-time cold chain monitoring system which can monitor your temperature-controlled shipments in-transit as well as in a warehouse.
HENGKO wireless temperature and humidity data logger adopts high precision sensor provide meaningful support at the highest technical level so that you are always able to optimally meet all of the legal specifications with your cold chain and process monitoring in this context!
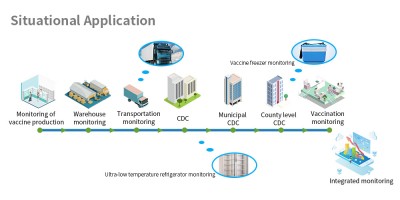
Real-time control of vehicle dynamics, automatic data storage and uploading to the cloud, real-time temperature and humidity monitoring. Real-time control of vehicle dynamics, automatic data storage and uploading to the cloud, real-time temperature and humidity monitoring. HENGKO IoT Intelligent temperature condition monitoring provides the tools needed to preserve stock in a fully automated cloud-based system. A simple to use solution, right-out-of-the-box, that monitor status using a configurable software and application builder: Android APP, WeChat small program , WeChat public number and PC. Save time and add efficiency for your reporting requirements.
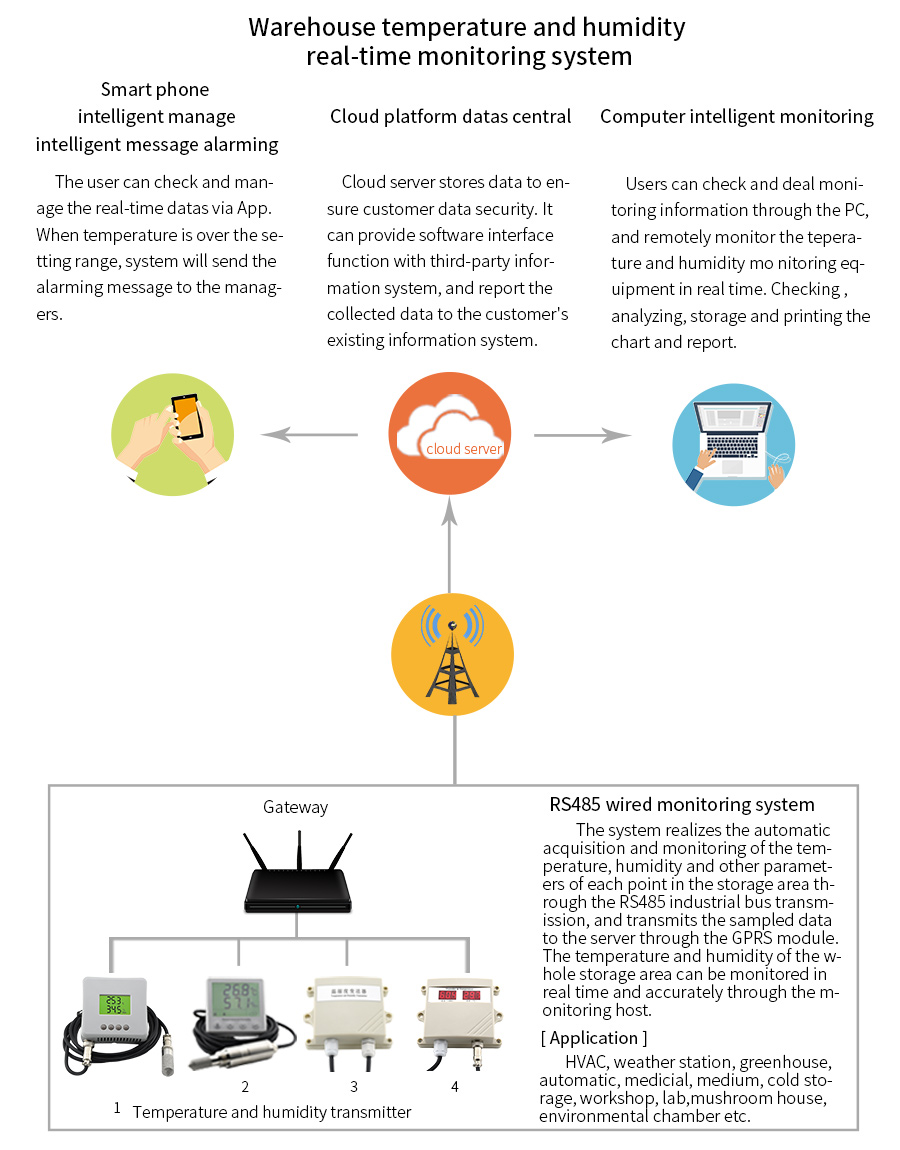
Temperature indicators and recorders introduce accountability measures into the shipping and handling stages of the temperature sensitive cold chain. In the event that a temperature excursion occurs, indicators and recorders give you the data needed to improve the integrity of the cold chain.
Temperature monitoring and measuring devices play a vital role in identifying temperature-related events, and empower you to take meaningful action to reduce the likelihood of product damage.
The Ins and Outs of the Cold Chain Process
1. The Manufacturing Stage
The journey of a vaccine begins in the lab, where scientists and researchers work diligently to create these life-saving formulas. Once the vaccines are produced, they're immediately placed in a temperature-controlled environment.
2. Distribution: From the Lab to the Field
This is where the real test for the cold chain starts. Vaccines need to be transported from the manufacturer to the end user, often traveling through various geographic and climatic zones. Each step of this process – from the manufacturer to the distribution center, from the distribution center to the healthcare provider, and finally, to the patient – requires meticulous temperature control and monitoring.
3. Vaccination: The Final Frontier
The final link in the cold chain is the healthcare providers administering the vaccines. It’s crucial they follow the correct storage protocols until the vaccine is administered to ensure its effectiveness.
Common Challenges in Maintaining Cold Chain Integrity
1. Geographic and Climatic Challenges
One of the main issues in maintaining the cold chain is the wide range of climates and geographic terrains vaccines must traverse. Remote regions pose unique challenges, including unreliable electricity supply, extreme temperatures, and difficult terrains.
2. Logistical and Infrastructure Challenges
Transporting vaccines can be a logistical nightmare, especially in areas with inadequate infrastructure. Problems like lack of reliable transport, inadequate storage facilities, and scarcity of trained personnel can all hinder cold chain integrity.
3. The Risk of Human Error
Even with the best systems in place, human error can disrupt the cold chain. Simple mistakes, like not properly closing a refrigerator door, can expose vaccines to unsuitable temperatures, compromising their efficacy.
The Role of Technology in Cold Chain Monitoring
Temperature Monitoring Devices
The advent of technology has been a game-changer in ensuring cold chain integrity. For example, temperature monitoring devices can track the temperature of vaccines in real time during transport and storage, alerting handlers if there's a breach in the required temperature range.
1. Digital Data Loggers
Another key player in the technological ensemble are digital data loggers. These devices can record temperature data over time, providing a comprehensive view of the temperature conditions the vaccines have been exposed to.
2. Internet of Things (IoT) in Cold Chain Management
IoT technology has the potential to revolutionize the cold chain by connecting all stages of vaccine distribution, providing real-time visibility and control over the process. IoT can enable continuous monitoring, quick response to issues, and predictive analytics for future planning.
The Human Element in Vaccine Storage and Distribution
1. Training and Education
While technology plays a significant role, it's the people behind the machines who make the real difference. Training and education are critical to maintaining cold chain integrity. From the scientists in the lab to the health workers in the field, everyone must understand the importance of their role in preserving vaccine efficacy.
3. Building Strong Teams
Effective cold chain management also requires strong teamwork. All players – manufacturers, logistics providers, healthcare providers, and regulatory authorities – need to collaborate to ensure the vaccines' safe journey.
4. Public Participation
The public also plays an important role in this complex journey. Their understanding of vaccine storage practices can aid in ensuring effective immunization campaigns.
Future Trends and Predictions for Vaccine Cold Chain Management
1. Emergence of Next-Generation Vaccines
With the development of novel vaccines, like mRNA vaccines, that require ultra-low temperatures, the importance of the cold chain is set to increase. These next-gen vaccines may necessitate an overhaul of current cold chain systems.
2. Innovation in Cold Chain Technologies
Expect to see more technological advancements aimed at enhancing cold chain management. These could include AI-powered predictive analytics for cold chain logistics, blockchain for improved transparency, and drones for delivering vaccines to remote areas.
3. Increased Investment in Infrastructure
As the demand for vaccines increases, so will the need for robust cold chain infrastructure. Expect significant investments in this area, especially in developing regions.
FAQs
What is the cold chain in vaccine storage?
The cold chain refers to the continuous process of storing and transporting vaccines at recommended temperatures from the point of manufacturing to the point of use.
Why is the cold chain important for vaccine integrity?
The cold chain is critical for maintaining vaccine efficacy. Most vaccines need to be kept within a specific temperature range to maintain their potency.
What are the challenges in maintaining cold chain integrity?
Challenges in maintaining cold chain integrity include varying geographical and climatic conditions, infrastructure and logistical issues, and human error.
How does technology aid in cold chain management?
Technology aids in cold chain management through temperature monitoring devices, digital data loggers, and Internet of Things (IoT) connectivity. These advancements allow for real-time tracking, recording, and analysis of temperature conditions throughout the vaccine distribution process.
What is the role of humans in the vaccine cold chain?
Humans play a vital role in the vaccine cold chain. From those involved in manufacturing, transport, and administration, to the end recipients of the vaccine, each person's awareness and actions can impact the integrity of the cold chain.
What are future trends in vaccine cold chain management?
Future trends include the development of next-generation vaccines requiring ultra-cold storage, the emergence of innovative cold chain technologies, and increased investments in cold chain infrastructure.
Conclusion
The Complex Journey of Vaccine Storage: Ensuring Cold Chain Integrity is a mammoth task that requires meticulous planning, advanced technology, and rigorous training. Each link in this cold chain is crucial to ensure vaccines retain their potency from the lab to the patient's arm.
Despite the challenges, the importance of maintaining cold chain integrity cannot be overstated. With the global health at stake, and in the light of emerging vaccination demands, continuous improvement and investment in cold chain systems are a public health priority.
As the saying goes, a chain is only as strong as its weakest link. In the journey of vaccine storage and distribution, there's no room for a weak link. Here's to a world where every vaccine's journey is a successful one, contributing to the global fight against infectious diseases.