There is No Slacking Off in the Vaccine Transport

The COVID-19 vaccination has been in full swing recently. Have everyone been vaccinated against the COVID-19 vaccine? Vaccines are divided into live vaccines and dead vaccines. Commonly used live vaccines include BCG, polio vaccine, measles vaccine, and plague vaccine. As a special medicine, the circulation process of vaccines can be roughly divided into three links: procurement, transportation, and traceability. Each link is more stringent than ordinary drugs.
Unified Procurement Channels
In 2019, Chinese newly promulgated the "Vaccine Management Law of the People's Republic of China" (hereinafter referred to as the "Vaccine Management Law"), which stipulates that the national immunization program vaccines shall be formed through centralized bidding or unified negotiation by the health authority of the State Council and the financial department of the State Council.
And announce the winning bid price or transaction price, all provinces, autonomous regions, and municipalities directly under the Central Government implement unified procurement. The end of the vaccine circulation is the inoculation points of the Centers for Disease Control and Prevention (CDC) and the primary health service centers at all levels.
Since the first-type seedlings are inoculated by the state and do not require market promotion, they are uniformly purchased by the government on the provincial platform and distributed directly by the manufacturer to the terminal CDC.
Strict Storage and Transportation
Different types of vaccines require different storage conditions. It must be stored in accordance with the conditions specified in the instructions to ensure that the vaccine will not "deteriorate".
Generally speaking, inactivated vaccines should be stored in an environment of 2 to 8°C and protected from light, and should not be frozen. Live vaccines should generally be stored in the dark under the condition of -15℃d1. For freeze-dried attenuated live virus (bacteria) vaccines, in addition to individual requirements, cryopreservation is generally required, and the storage temperature should be stable, especially not repeated Freeze-thaw.
Therefore, vaccine storage and transportation emphasize cold chain management. From manufacturers to inoculation units, vaccines are required to be stored, transported and used under specified temperature conditions, that is, to ensure uninterrupted cold chain throughout the entire process, real-time monitoring, and formulate transportation plans in advance.
What HENGKO Can Do For You ?
The measurement accuracy required by automatic temperature monitoring equipment during vaccine storage and transportation is within ±0.5°C. HENGKO Hk-J9A100 series temperature and humidity data logger is a new generation of temperature and humidity recording products. It adopts high precision sensor.
The error range is within ±0.3℃. And the time interval set by the user automatically stores data (1s~24 hours), equipped with intelligent data analysis and management software, to provide users with long-term, professional temperature and humidity measurement, recording, alarm, analysis, etc., to meet customers' temperature Different application requirements for humidity sensitive occasions.
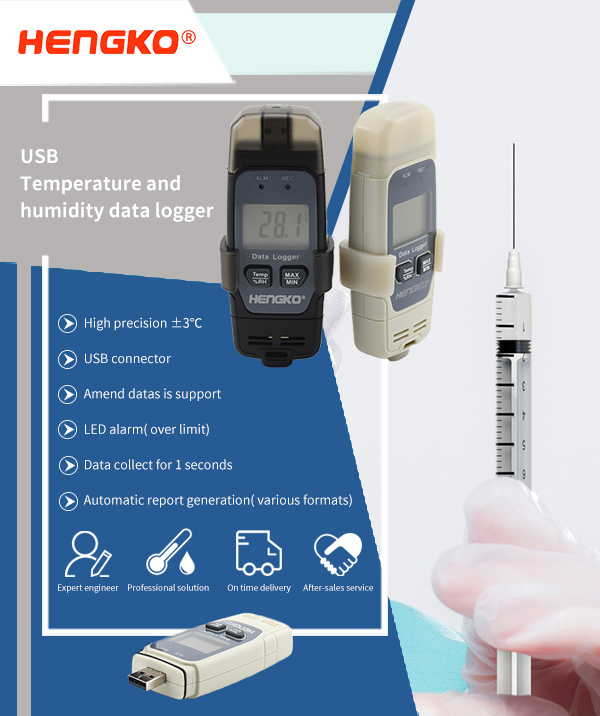
Regardless of whether the vaccine is stored or transported, the vaccine distribution companies, disease prevention and control agencies, and vaccination units are required to monitor the temperature, and fill in the "vaccine transportation temperature record form" with a recording interval of no less than 6 hours.
Therefore, during the cold chain transportation of vaccines, the control of temperature and humidity is very important, and the construction of a temperature and humidity monitoring system for vaccine transportation is essential.
HENGKO has been deeply involved in the temperature and humidity instrument industry for many years, and our products have been exported to Europe, the United States, and Japan for a long time. , Russia, Canada, Australia, Southeast Asia and other developed industrial economies that have high-quality requirements for products in this industry.
Scientific Traceability System
At present, the national free vaccines are received and distributed through the distribution channels of “provincial → municipal → districts and counties → inoculation units and township medical and health institutions” to ensure that they are within the management of the provincial CDC, and the vaccine starts from reaching the provincial CDC Until the vaccine is inoculated to the recipient, the traceability of vaccine information in the whole process is realized to ensure the quality control and safety of the vaccine.
Vaccines are special medicines, and the strict transportation management system guarantees the stability and effectiveness of the vaccine quality and puts a "safety lock" on our vaccination.
Contact HENGKO to Upgrade Quality of Vaccine Transport
With the ongoing global pandemic, it's crucial to ensure safe and efficient vaccine transport. At HENGKO, we understand the critical role our products play in this process. Our sintered metal filters and gas filtration products are designed to ensure the safe and reliable transport of vaccines, helping to protect public health and save lives. Contact us today to learn more about our products and how they can support your vaccine transport needs.
If you're looking for precise temperature and humidity monitoring, HENGKO's sensors are the perfect solution. Our team of experts has extensive experience in the field and is equipped to provide high-quality sensor solutions that are tailored to meet the specific needs of each client. We work closely with each client to ensure that their sensor solution is designed and manufactured to meet their specific requirements.
